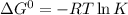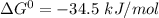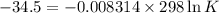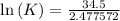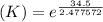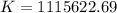Determine the equilibrium constant for the following reaction at 298 k.
cl(g) + o3(g) → clo(g)...

Chemistry, 20.12.2019 18:31 alexabbarker9781
Determine the equilibrium constant for the following reaction at 298 k.
cl(g) + o3(g) → clo(g) + o2(g)
δg° = - 34.5 kj

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1


Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
Questions

Mathematics, 20.10.2019 05:50




Health, 20.10.2019 05:50

Mathematics, 20.10.2019 05:50




Social Studies, 20.10.2019 05:50



Geography, 20.10.2019 05:50



English, 20.10.2019 05:50

Mathematics, 20.10.2019 05:50