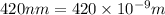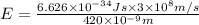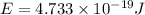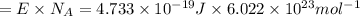Chemistry, 20.12.2019 18:31 pablogonzaleztellez
An important reaction in the formation of photochemical smog is the photodissociation of no2: no2 + hv > > > no(g) + o(g) the maximum wavelength of light that can cause this reaction is 420 nm. a) in what part of the electromagnetic spectrum is light with this wavelength found? b) what is the maximum strength of a bond, in kj/mol, that can be broken by absorption of a photon of 420-nm light? c) write out the photodissociation reaction showing lewis-dot structures.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 23.06.2019 10:30
How much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 3
You know the right answer?
An important reaction in the formation of photochemical smog is the photodissociation of no2: no2 +...
Questions


History, 13.07.2019 11:00

Geography, 13.07.2019 11:00

Spanish, 13.07.2019 11:00

Mathematics, 13.07.2019 11:00


Social Studies, 13.07.2019 11:00

History, 13.07.2019 11:00


Health, 13.07.2019 11:00




Mathematics, 13.07.2019 11:00





Arts, 13.07.2019 11:00

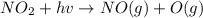
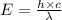
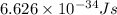
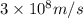
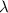 = wavelength =
= wavelength =