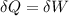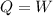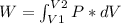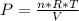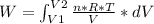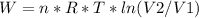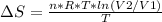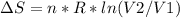Chemistry, 20.12.2019 19:31 Mattixwillard
An ideal gas contained in a piston-cylinder assembly is compressed isothermally in an internally reversible process.
(a) determine if the entropy change of the gas is greater than, equal to or less than zero, justify your answer
(b) determine if for the same change of state, the entropy change for an irreversible process is greater than, equal to or less than part (a)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
An ideal gas contained in a piston-cylinder assembly is compressed isothermally in an internally rev...
Questions

English, 25.02.2021 22:10

Chemistry, 25.02.2021 22:10


Mathematics, 25.02.2021 22:10



Health, 25.02.2021 22:10


Computers and Technology, 25.02.2021 22:10


Mathematics, 25.02.2021 22:10

Chemistry, 25.02.2021 22:10


Mathematics, 25.02.2021 22:10


Mathematics, 25.02.2021 22:10


Mathematics, 25.02.2021 22:10


Mathematics, 25.02.2021 22:10

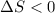
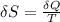
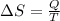
![\delta U=[tex]\delta Q- \delta W](/tpl/images/0427/9845/795a2.png)
![0=[tex]\delta Q- \delta W](/tpl/images/0427/9845/24655.png)