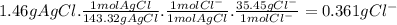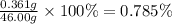To measure the amount of chlorine in a well-boring fluid, an analytical chemist adds 0.1600 m silver nitrate (agno3) solution to a 46.00 g sample of the fluid and collects the solid silver chloride (agcl) product. when no more agcl is produced, he filters, washes and weighs it, and finds that 1.46 g has been produced.
the balanced chemical equation for the reaction is:
cl^- (aq) + agno3(aq) > agcl(s) + no3^- (aq)
1. what kind of reaction is this?
o precipitation o acid-base o redox
2. if you said this was a precipitation reaction, enter the chemical formula of the precipitate.
3. if you said this was an acid-base reaction, enter the chemical formula of the reactant that is acting as the base.
4. if you said this was a redox reaction, enter the chemical symbol of the element that is oxidized.
5. calculate the mass percent of cl in the sample. be sure your answer has the correct number of significant digits.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1


Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
To measure the amount of chlorine in a well-boring fluid, an analytical chemist adds 0.1600 m silver...
Questions

Mathematics, 22.04.2021 04:50

Mathematics, 22.04.2021 04:50


Mathematics, 22.04.2021 04:50



Physics, 22.04.2021 04:50

History, 22.04.2021 04:50


Mathematics, 22.04.2021 04:50



Social Studies, 22.04.2021 04:50


Mathematics, 22.04.2021 04:50

English, 22.04.2021 04:50



Mathematics, 22.04.2021 04:50