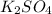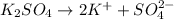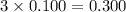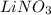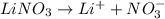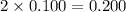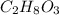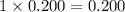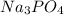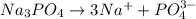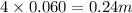Chemistry, 20.12.2019 20:31 bronkosarecool
Choose the aqueous solution that has the highest boiling point. these are all solutions of nonvolatile solutes and you should assume ideal van't hoff factors where applicable. a. 0.100 m k2so4 b. 0.100 m lino3 c. 0.200 m c3h8o3 d. 0.060 m na3po4 e. they all have the same boiling point.

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 20:30
After cloud droplets form, what must happen to them for precipitation to occur?
Answers: 1

Chemistry, 22.06.2019 01:30
100 points answer quick the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1
You know the right answer?
Choose the aqueous solution that has the highest boiling point. these are all solutions of nonvolati...
Questions




Mathematics, 05.11.2020 19:50


Biology, 05.11.2020 19:50


History, 05.11.2020 19:50


History, 05.11.2020 19:50





Mathematics, 05.11.2020 19:50

Mathematics, 05.11.2020 19:50

Physics, 05.11.2020 19:50



Business, 05.11.2020 19:50

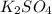
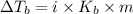
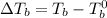 = Elevation in boiling point
= Elevation in boiling point 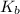 = boiling point constant
= boiling point constant