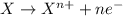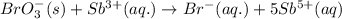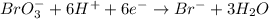Chemistry, 20.12.2019 21:31 holaadios222lol
Complete and balance the following redox equation using the set of smallest whole-number coefficients. now sum the coefficients of all species in the balanced equation. (remember the coefficients that are equal to one.) bro3-(aq) + sb3+(aq) ? br-(aq) + sb5+(aq) (acid solution) the sum of the coefficients is

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3
You know the right answer?
Complete and balance the following redox equation using the set of smallest whole-number coefficient...
Questions




Chemistry, 07.10.2019 03:00



Mathematics, 07.10.2019 03:00





Biology, 07.10.2019 03:00

English, 07.10.2019 03:00







Biology, 07.10.2019 03:00