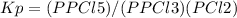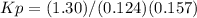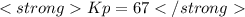Chemistry, 20.12.2019 21:31 BLASIANNkidd
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction:
pcl3 (g) + cl2 (g) → pcl5 (g)
an equilibrium mixture at 450 k contains ppcl3 = 0.124 atm, pcl2 = 0.157 atm, and ppcl5 = 1.30 atm.
what is the value of kp at this temperature?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlor...
Questions

Spanish, 13.10.2019 18:30

Advanced Placement (AP), 13.10.2019 18:30




Arts, 13.10.2019 18:30

Mathematics, 13.10.2019 18:30

Mathematics, 13.10.2019 18:30

Chemistry, 13.10.2019 18:30


English, 13.10.2019 18:30



English, 13.10.2019 18:30


Biology, 13.10.2019 18:30

Mathematics, 13.10.2019 18:30

Mathematics, 13.10.2019 18:30

Biology, 13.10.2019 18:30