
Chemistry, 20.12.2019 23:31 andreamartinez7876
Use mathematical software or an electronic spreadsheet to examine thetime dependence of [i] in the reaction mechanism a →i→p (k1,k2). youmay either integrate eqn 22.39 numerically (seeappendix2) or use eqn 22.40directly. in all the following calculations, use [a]0=1 mol dm−3and a timerange of 0 to 5 s. (a) plot [i] against tfork1=10 s−1andk2=1 s−1. (b) increasethe ratio k2/k1steadily by decreasing the value of k1and examine the plot of [i]againsttat each turn. what approximation about d[i]/dtbecomesincreasingly valid?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Embryos of different species look very similar, which shows that the organisms share a ancestor.
Answers: 1

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
Use mathematical software or an electronic spreadsheet to examine thetime dependence of [i] in the r...
Questions


Mathematics, 30.01.2021 09:20

Mathematics, 30.01.2021 09:20

Mathematics, 30.01.2021 09:20

English, 30.01.2021 09:20

English, 30.01.2021 09:20


History, 30.01.2021 09:20

Biology, 30.01.2021 09:20


Mathematics, 30.01.2021 09:20



Mathematics, 30.01.2021 09:20

Business, 30.01.2021 09:20





English, 30.01.2021 09:20

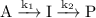
![\text{[I]} = \dfrac{k_{1}\text{[A]}_{0}}{k_{2} - k_{1}} \left(e^{-k_{1}t} - e^{-k_{2}t} \right )](/tpl/images/0428/3191/dc9ab.png)
![\text{[I]} = \dfrac{10}{-9} \left(e^{-10t} - e^{-t} \right )](/tpl/images/0428/3191/34abe.png)
![\mathbf{\dfrac{\text{d[I]}}{\text{d}t}=0}](/tpl/images/0428/3191/041dc.png)
![Use mathematical software or an electronic spreadsheet to examine thetime dependence of [i] in the r](/tpl/images/0428/3191/f5590.jpg)
![Use mathematical software or an electronic spreadsheet to examine thetime dependence of [i] in the r](/tpl/images/0428/3191/65bc4.jpg)


