
Chemistry, 20.12.2019 23:31 michaelchavez6959127
In a different experiment, a student places a piece of pure ba(s) in a beaker containing 250.ml of 6.44mhcl(aq) and observes that the rate of appearance of h2(g) bubbles when ba(s) was added is much faster than the rate of appearance of bubbles when the mg(s) was added. the student claims that the greater reactivity is due to the difference in ionization energy between mg and ba. explain the difference in ionization energy between mg and ba in terms of atomic structure.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

You know the right answer?
In a different experiment, a student places a piece of pure ba(s) in a beaker containing 250.ml of 6...
Questions


Advanced Placement (AP), 31.07.2019 14:00


Social Studies, 31.07.2019 14:00


Biology, 31.07.2019 14:00




Physics, 31.07.2019 14:00




English, 31.07.2019 14:00






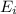
![[Ne]3s^2](/tpl/images/0428/3026/d3dcb.png)
![[Xe]6s^2](/tpl/images/0428/3026/f6f70.png)


