
Chemistry, 21.12.2019 00:31 wolfgirl2431
Achemical reaction turns substance a into substance b. the rate at which the reaction takes place is proportional to the square root of the amount of substance a present.
if is the amount of substance a present (measured in grams) at any time (measured in minutes), set up a differential equation for in terms of . if there is an arbitrary constant in your equation, call it and make sure that > 0k> 0.
now, suppose that the chemical reaction is to take place in a large tank of liquid!
the tank contains 25 liters of a solution of substance a dissolved in water. the concentration of the solution is 750 grams per liter. a 200 gram per liter solution of substance a is poured in at 12 liters per minute. at the same time, the solution is drained from the bottom of the tank at 11 liters per minute.
if is the amount of substance a in the tank (measured in grams) at any time (measured in minutes), set up a differential equation for in terms of :

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1
You know the right answer?
Achemical reaction turns substance a into substance b. the rate at which the reaction takes place is...
Questions

Mathematics, 20.09.2019 02:00



Mathematics, 20.09.2019 02:10

Mathematics, 20.09.2019 02:10




Mathematics, 20.09.2019 02:10


Mathematics, 20.09.2019 02:10

Mathematics, 20.09.2019 02:10


Mathematics, 20.09.2019 02:10

Social Studies, 20.09.2019 02:10

History, 20.09.2019 02:10




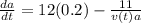
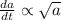
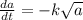
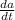 will be high
will be high
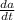 = 12 (200 grams) - 11/(v(t))a
= 12 (200 grams) - 11/(v(t))a



