
Chemistry, 21.12.2019 02:31 leeahnnfoster
Anabolic steroids are sometimes used illegally by athletes to increase muscle strength. a forensic chemist analyzes some tablets suspected of being a popular steroid.
he determines the substance contains only c, h and o and has a molar mass of 300.42g/mol.
when a 1.200g sample is subjected to combustion analysis, 3.516g of co2 and 1.007g h2o are collected. a) what is the empirical formula of the unknown substance in the tablets? b)what is the molecular formula of the unknown substance in the tablets?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2
You know the right answer?
Anabolic steroids are sometimes used illegally by athletes to increase muscle strength. a forensic c...
Questions

Mathematics, 24.07.2019 19:00


Mathematics, 24.07.2019 19:00



Mathematics, 24.07.2019 19:00

History, 24.07.2019 19:00

English, 24.07.2019 19:00

Biology, 24.07.2019 19:00




Mathematics, 24.07.2019 19:00

Mathematics, 24.07.2019 19:00




Mathematics, 24.07.2019 19:00


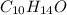
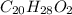
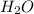 = 1.007 g /18 g/mol = 0.0559 moles
= 1.007 g /18 g/mol = 0.0559 moles
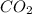 = 3.516 g /44.01 g/mol = 0.0799 moles
= 3.516 g /44.01 g/mol = 0.0799 moles


