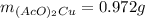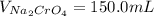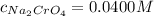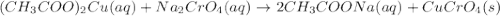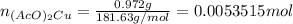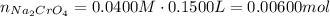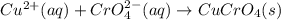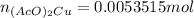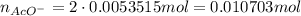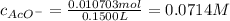Chemistry, 21.12.2019 05:31 nataliemoore1974
Suppose of copper(ii) acetate is dissolved in of a aqueous solution of sodium chromate. calculate the final molarity of acetate anion in the solution. you can assume the volume of the solution doesn't change when the copper(ii) acetate is dissolved in it. be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 16:00
What is the cellular process that releases the energy stored in food molecules
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
Suppose of copper(ii) acetate is dissolved in of a aqueous solution of sodium chromate. calculate th...
Questions


History, 04.09.2020 03:01



English, 04.09.2020 03:01

History, 04.09.2020 03:01




Biology, 04.09.2020 03:01

Social Studies, 04.09.2020 03:01

Physics, 04.09.2020 03:01


Mathematics, 04.09.2020 03:01

Mathematics, 04.09.2020 03:01

Mathematics, 04.09.2020 03:01

Engineering, 04.09.2020 03:01

Mathematics, 04.09.2020 03:01

History, 04.09.2020 03:01

Computers and Technology, 04.09.2020 03:01