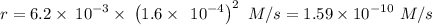The second-order rate constant for the dimerization of a protein (p) p + p → p2 is 6.2 × 10−3/m · s at 25°c. part 1 out of 2 if the concentration of the protein is 1.6 × 10−4 m, calculate the initial rate (m/s) of formation of p2. rate = × 10 m/s (enter your answer in scientific notation.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
How much energy is made when a pice of wood burns. how do you know
Answers: 2

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2


Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
The second-order rate constant for the dimerization of a protein (p) p + p → p2 is 6.2 × 10−3/m · s...
Questions

Mathematics, 08.11.2020 17:00

Mathematics, 08.11.2020 17:00

Mathematics, 08.11.2020 17:00

Physics, 08.11.2020 17:00


Mathematics, 08.11.2020 17:00

Geography, 08.11.2020 17:00





Mathematics, 08.11.2020 17:00



English, 08.11.2020 17:00

Health, 08.11.2020 17:00

Mathematics, 08.11.2020 17:00

Chemistry, 08.11.2020 17:00


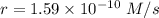
![r=k[P]^2](/tpl/images/0428/7363/45884.png)
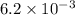 /Ms
/Ms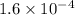 M
M