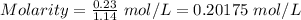Chemistry, 21.12.2019 05:31 snikergrace
At a particular temperature, k = 1.00×102 for the reaction: h2(g) + f2(g)= 2hf(g) in an experiment, at this temperature, 1.40 mol of h2 and 1.40 mol of f2 are introduced into a 1.14-l flask and allowed to react. at equilibrium, all species remain in the gas phase. what is the equilibrium concentration (in mol/l) of h2?

Answers: 2
Another question on Chemistry



Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
At a particular temperature, k = 1.00×102 for the reaction: h2(g) + f2(g)= 2hf(g) in an experiment,...
Questions

English, 22.01.2021 06:40





Mathematics, 22.01.2021 06:40

Social Studies, 22.01.2021 06:40


Mathematics, 22.01.2021 06:40


Mathematics, 22.01.2021 06:40

Mathematics, 22.01.2021 06:40



Health, 22.01.2021 06:40



Physics, 22.01.2021 06:40

Mathematics, 22.01.2021 06:40

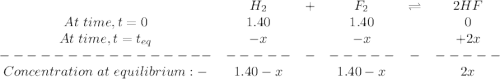
![K_c=\frac{[HF]^2}{[H_2][F_2]}](/tpl/images/0428/7279/a2854.png)
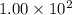
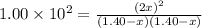
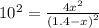
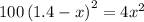
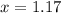
![[H_2]_{eq}=1.40-x=1.40-1.17=0.23\ moles](/tpl/images/0428/7279/ecf31.png)