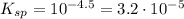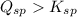2-

Chemistry, 21.12.2019 10:31 haylie1456
Q10.an aqueous solution contains 5.00x10-2 mol/l of ca2+ and 7.00x10-3 mol/l of so4
2-
. show whether
this solution would precipitate anhydrite caso4 or not provided that the ksp for anhydrite is equal to 10-4.5.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1


Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

You know the right answer?
Q10.an aqueous solution contains 5.00x10-2 mol/l of ca2+ and 7.00x10-3 mol/l of so4
2-
2-
Questions

Mathematics, 25.02.2021 21:30



Biology, 25.02.2021 21:30

Mathematics, 25.02.2021 21:30

Mathematics, 25.02.2021 21:30


Mathematics, 25.02.2021 21:30

Social Studies, 25.02.2021 21:30

Mathematics, 25.02.2021 21:30

English, 25.02.2021 21:30

Mathematics, 25.02.2021 21:30

Mathematics, 25.02.2021 21:30



Geography, 25.02.2021 21:30



Mathematics, 25.02.2021 21:30

 ⇄
⇄ 
![K_{sp}=[Ca^{2+}][SO_4^{2-}]=10^{-4.5}](/tpl/images/0428/9357/09b0c.png)
![Q_{sp}=[Ca^{2+}][SO_4^{2-}]=5.00\cdot10^{-2} M\cdot7.00\cdot10^{-3} M=3.5\cdot10^{-4}](/tpl/images/0428/9357/06e2a.png)