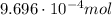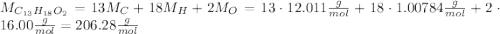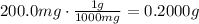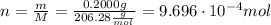Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:30
Which type of stress results when two plates push against one another? a. compression b. tension c. force d. shear
Answers: 1


Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1
You know the right answer?
ibuprofen (c13h1802) is the active ingredient in many nonprescription pain relievers. each tablet co...
Questions




Social Studies, 24.02.2020 23:08


Mathematics, 24.02.2020 23:08










History, 24.02.2020 23:08

History, 24.02.2020 23:09



Mathematics, 24.02.2020 23:09