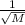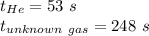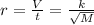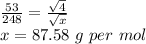Chemistry, 23.12.2019 18:31 22MadisonT
Asample of he gas (2.0 mmol) effused through a pinhole in 53 s. the same amount of an unknown gas, under the same conditions, effused through the pinhole in 248 s. the molecular mass of the unknown gas is g/mol.

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 04:50
The diagin dilutepage 6 of 12a6a5(a)fluorine, chlorine, bromine and iodine are placed in the same group of theperiodic table.state the common name used to describe elements in this group.(i)state the group in which the elements are placed and explain whythey are placed in that group.(ii)which of the above named elements is a solid at roomtemperature and pressure?
Answers: 2

Chemistry, 23.06.2019 11:30
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 23.06.2019 22:00
Suggest which functional groups are responsible for the reactions with(i) sodium,(ii) bromine water(iii) alkaline aqueous iodine.(3 marks)(iv) suggest structures for x and y.(1 mark)(b) one molecule that has been found in the dust clouds is hydroxyethanal: hoch2cho.name the two functional groups present in this compound.(2 marks)(c) but-1-ene, ch3ch2ch=ch2, is an important compound in the petrochemical industry.some reactions of but-l-ene are given below. in each empty box, draw the structural formulaand write the name of the organic compounds formed.
Answers: 3
You know the right answer?
Asample of he gas (2.0 mmol) effused through a pinhole in 53 s. the same amount of an unknown gas, u...
Questions




History, 26.09.2019 10:10


Mathematics, 26.09.2019 10:10


Computers and Technology, 26.09.2019 10:10

English, 26.09.2019 10:10

Chemistry, 26.09.2019 10:10


Mathematics, 26.09.2019 10:10


Mathematics, 26.09.2019 10:10


Mathematics, 26.09.2019 10:10


Social Studies, 26.09.2019 10:10


Mathematics, 26.09.2019 10:10