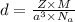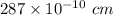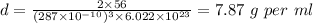Chemistry, 23.12.2019 19:31 laurentsupreme
Iron crystallizes in a body-centered cubic unit cell. the edge length of this cell is 287 pm. calculate the density of iron in g/ml.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 13:00
In a copper wire, a temperature increase is the result of which of the following
Answers: 1

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
Iron crystallizes in a body-centered cubic unit cell. the edge length of this cell is 287 pm. calcul...
Questions

Biology, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00


Spanish, 19.02.2021 01:00




Mathematics, 19.02.2021 01:00


English, 19.02.2021 01:00

Mathematics, 19.02.2021 01:00






Computers and Technology, 19.02.2021 01:00

Health, 19.02.2021 01:00