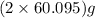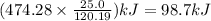Chemistry, 23.12.2019 19:31 DuckieTime
The thermochemical equation for the combustion of rubbing alcohol is 2c3h7oh (l) + 9o2 → 6co2 +8h2o(l) δh = -474.8 kj calculate the amount of heat released when 25.0 g of rubbing alcohol is combusted.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
The thermochemical equation for the combustion of rubbing alcohol is 2c3h7oh (l) + 9o2 → 6co2 +8h2o(...
Questions

History, 02.02.2021 03:10



History, 02.02.2021 03:10





Mathematics, 02.02.2021 03:10

Social Studies, 02.02.2021 03:10

Mathematics, 02.02.2021 03:10






Mathematics, 02.02.2021 03:10

History, 02.02.2021 03:10


Mathematics, 02.02.2021 03:10

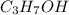 (rubbing alcohol) is combusted equal to 98.7 kJ
(rubbing alcohol) is combusted equal to 98.7 kJ