
Chemistry, 23.12.2019 20:31 serenityarts123
Consider an ideal gas enclosed in a 1.00 l container at an internal pressure of 18.0 atm. calculate the work, w , if the gas expands against a constant external pressure of 1.00 atm to a final volume of 18.0 l.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1


Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3

Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
Consider an ideal gas enclosed in a 1.00 l container at an internal pressure of 18.0 atm. calculate...
Questions





Chemistry, 15.10.2020 07:01


Mathematics, 15.10.2020 07:01


English, 15.10.2020 07:01



Mathematics, 15.10.2020 07:01


Business, 15.10.2020 07:01


Chemistry, 15.10.2020 07:01


Physics, 15.10.2020 07:01


Health, 15.10.2020 07:01

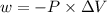
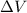 is the change in volume
is the change in volume
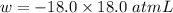
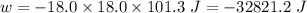 (negative sign implies that work is done by the system)
(negative sign implies that work is done by the system)

