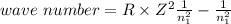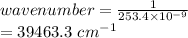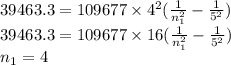Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

You know the right answer?
One of the emission spectral lines for be3+ has a wavelength of 253.4 nm for an electronic transitio...
Questions




Mathematics, 24.07.2019 05:30





Social Studies, 24.07.2019 05:30


Mathematics, 24.07.2019 05:30







Mathematics, 24.07.2019 05:30

Mathematics, 24.07.2019 05:30

Mathematics, 24.07.2019 05:30