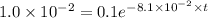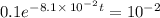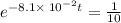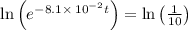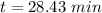Chemistry, 24.12.2019 00:31 desotoaustin
It is found that a gas undergoes a first-order decomposition reaction. if the rate constant for this reaction is 8.1 x 10-2 /min, how long will it take for the concentration of the gas to change from an initial concentration of .1m to 1.0 x 10-2 m?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
It is found that a gas undergoes a first-order decomposition reaction. if the rate constant for this...
Questions






Mathematics, 22.09.2020 14:01

Mathematics, 22.09.2020 14:01

Mathematics, 22.09.2020 14:01

Mathematics, 22.09.2020 14:01


Mathematics, 22.09.2020 14:01



Mathematics, 22.09.2020 14:01

Mathematics, 22.09.2020 14:01

Mathematics, 22.09.2020 14:01

Mathematics, 22.09.2020 14:01



History, 22.09.2020 14:01

![[A_t]=[A_0]e^{-kt}](/tpl/images/0431/0820/1ef89.png)
![[A_t]](/tpl/images/0431/0820/5262c.png) is the concentration at time t
is the concentration at time t
![[A_0]](/tpl/images/0431/0820/9a686.png) is the initial concentration
is the initial concentration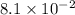 min⁻¹
min⁻¹
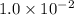 M
M