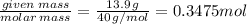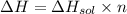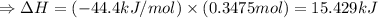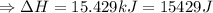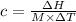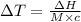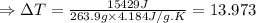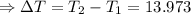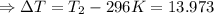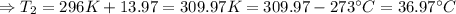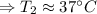Chemistry, 24.12.2019 02:31 coolman5999alt
The enthalpy of solution of sodium hydroxide is –44.4 kj/mol. when a 13.9-g sample of naoh dissolves in 250.0 g of water 23.0 °c in a coffee-cup calorimeter, what is the final temperature of the solution assuming no heat is lost to the surroundings. the solution has the same specific heat of 4.184 j/g-k.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3

Chemistry, 21.06.2019 22:00
Afamily is one another name for a group on the table of elements.
Answers: 1

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 09:50
Achemist has dissolved a certain substance in water. the chemist knows that more of the substance could be dissolved into the water before it stops dissolving. therefore
Answers: 2
You know the right answer?
The enthalpy of solution of sodium hydroxide is –44.4 kj/mol. when a 13.9-g sample of naoh dissolves...
Questions

Social Studies, 29.10.2020 09:40

Mathematics, 29.10.2020 09:40

Mathematics, 29.10.2020 09:40

History, 29.10.2020 09:40

Arts, 29.10.2020 09:40




Mathematics, 29.10.2020 09:40


Mathematics, 29.10.2020 09:40

Spanish, 29.10.2020 09:40



Mathematics, 29.10.2020 09:40

Mathematics, 29.10.2020 09:40

Mathematics, 29.10.2020 09:40



Mathematics, 29.10.2020 09:40

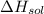 = – 44.4 kJ/mol,
= – 44.4 kJ/mol,