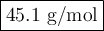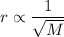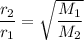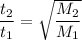Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3


Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Asample of an unknown gas takes 222 s to diffuse through a porous plug at a given temperature. at th...
Questions




Advanced Placement (AP), 13.08.2021 01:30

Physics, 13.08.2021 01:30


English, 13.08.2021 01:30





Computers and Technology, 13.08.2021 01:30

Social Studies, 13.08.2021 01:30

Mathematics, 13.08.2021 01:30



Computers and Technology, 13.08.2021 01:30