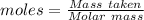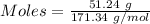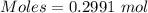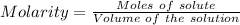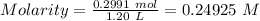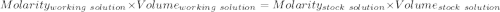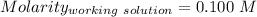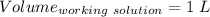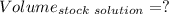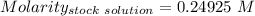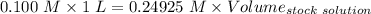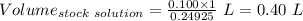Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3


Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
A51.24-g sample of ba(oh)2 is dissolved in enough water to make 1.20 liters of solution. how many ml...
Questions


English, 14.01.2021 17:40

Mathematics, 14.01.2021 17:40

Mathematics, 14.01.2021 17:40


Health, 14.01.2021 17:40

Mathematics, 14.01.2021 17:40



Social Studies, 14.01.2021 17:40

Mathematics, 14.01.2021 17:40





Mathematics, 14.01.2021 17:40

Chemistry, 14.01.2021 17:40



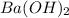 as:-
as:-