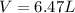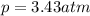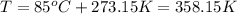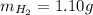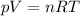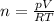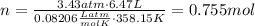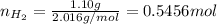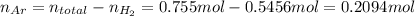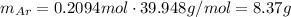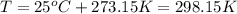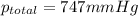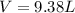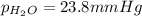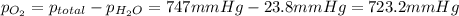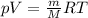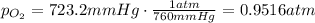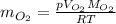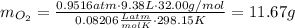Chemistry, 24.12.2019 18:31 yousifgorgees101
Amixture of hydrogen and argon gases is maintained in a 6.47 l flask at a pressure of 3.43 atm and a temperature of 85 °c. if the gas mixture contains 1.10 grams of hydrogen, the number of grams of argon in the mixture is g.
b) oxygen gas can be prepared by heating potassium chlorate according to the following equation:
2kclo3(s) > 2kcl(s) + 3o2(g)
the product gas, o2, is collected over water at a temperature of 25 °c and a pressure of 747 mm hg. if the wet o2 gas formed occupies a volume of 9.38l, the number of grams of o2 formed is g. the vapor pressure of water is 23.8 mm hg at 25 °c.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2
You know the right answer?
Amixture of hydrogen and argon gases is maintained in a 6.47 l flask at a pressure of 3.43 atm and a...
Questions

Mathematics, 27.03.2020 04:50







Physics, 27.03.2020 04:50

Mathematics, 27.03.2020 04:50

Mathematics, 27.03.2020 04:50






English, 27.03.2020 04:51