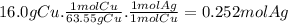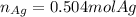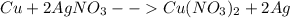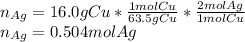Given the following reaction: \ce{cu + 2agno3 -> 2ag + cu(no3)2}cu+2agno3 2ag+cu(nox 3 )x 2 how many moles of \ce{ag}aga, g will be produced from 16.0 \text{ g}16.0 g16, point, 0, start text, space, g, end text of \ce{cu}cuc, u, assuming \ce{agno3}agno3 is available in excess

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 12:30
What type of scientific model does the flow chart represent? (a chart of the scientific process) a. conceptual b. mathematical c. physical d. virtual
Answers: 1

Chemistry, 21.06.2019 20:40
If equal masses of the listed metals were collected , which would have a greatest volume ? a. aluminum 2.70,b.zinc7.14,c.copper 8.92,d.lead 11.34
Answers: 2

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1
You know the right answer?
Given the following reaction: \ce{cu + 2agno3 -> 2ag + cu(no3)2}cu+2agno3 2ag+cu(nox 3 )x 2...
Questions

Mathematics, 01.02.2021 05:20


Mathematics, 01.02.2021 05:20


English, 01.02.2021 05:20


Mathematics, 01.02.2021 05:20

History, 01.02.2021 05:20



Mathematics, 01.02.2021 05:20

Mathematics, 01.02.2021 05:20


Mathematics, 01.02.2021 05:20

English, 01.02.2021 05:20



English, 01.02.2021 05:20

Mathematics, 01.02.2021 05:20

Mathematics, 01.02.2021 05:20