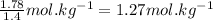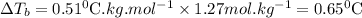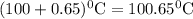Chemistry, 24.12.2019 20:31 tracyaleblanc
What is the boiling point of a solution produced by adding 610 g of cane sugar (molar mass 342.3 g/mol) to 1.4 kg of water? for each mole of nonvolatile solute, the boiling point of 1 kg of water is raised 0.51 ∘c.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 00:00
The pressure inside a hydrogen-filled container was 2.10 atm at 21 ? c. what would the pressure be if the container was heated to 92 ? c ?
Answers: 2

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
What is the boiling point of a solution produced by adding 610 g of cane sugar (molar mass 342.3 g/m...
Questions

Mathematics, 29.06.2019 00:30

Geography, 29.06.2019 00:30





Mathematics, 29.06.2019 00:30


Physics, 29.06.2019 00:30

Mathematics, 29.06.2019 00:30

English, 29.06.2019 00:30


Social Studies, 29.06.2019 00:30


Mathematics, 29.06.2019 00:30


English, 29.06.2019 00:30



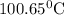
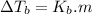
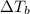 is elivation in boiling point of solution,
is elivation in boiling point of solution, 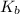 is ebbulioscopic constant of solvent (how much temperature is raised for dissolution of 1 mol of non-volatile solute) and m is molality of solution.
is ebbulioscopic constant of solvent (how much temperature is raised for dissolution of 1 mol of non-volatile solute) and m is molality of solution.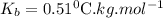
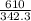 moles of cane sugar
moles of cane sugar