Chemistry, 24.12.2019 20:31 smartgirl61987
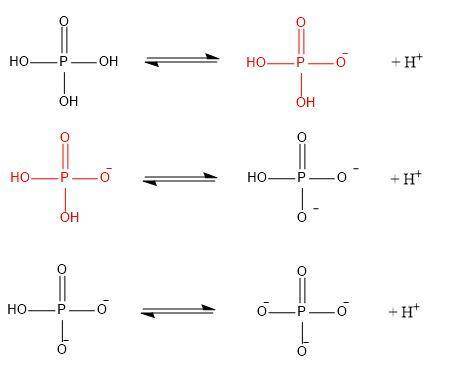
Phosphate is derived from the titration of phosphoric acid, h3po4. the three pka’s of h3po4 are 2.15, 7.20, and 12.35 respectively.
a/ write out the series of ionization (equilibrium) reactions corresponding to each ionization, making sure to write out the (flat) structure each molecule/ion as you do so. mark correct chemical bonds.
b/ in your diagram above, circle the dominant form of phosphate as it would appear at ph 5.7.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
Phosphate is derived from the titration of phosphoric acid, h3po4. the three pka’s of h3po4 are 2.15...
Questions


Mathematics, 29.06.2019 18:30

Biology, 29.06.2019 18:30


English, 29.06.2019 18:30

Mathematics, 29.06.2019 18:30


Mathematics, 29.06.2019 18:30

History, 29.06.2019 18:30


Mathematics, 29.06.2019 18:30


Mathematics, 29.06.2019 18:30


Computers and Technology, 29.06.2019 18:30

History, 29.06.2019 18:30

History, 29.06.2019 18:30

Spanish, 29.06.2019 18:30