
0.658 g of a compound containing only carbon, hydrogen, and oxygen is burned in excess o2. co2(1.285 g) and h20 (0.658g) are produced. the mol mass of the compound is determined by mass spectrometry to be 90 g/mole determine the empirical and molecular formulas. 20: 0-8453x(12.01s /uu. o 032 029/1b. a mal 1 2

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

You know the right answer?
0.658 g of a compound containing only carbon, hydrogen, and oxygen is burned in excess o2. co2(1.285...
Questions




Mathematics, 24.05.2021 14:00




Mathematics, 24.05.2021 14:00

Mathematics, 24.05.2021 14:00


Mathematics, 24.05.2021 14:00


Computers and Technology, 24.05.2021 14:00








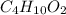
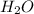 = 0.658 g /18 g/mol = 0.03656 moles
= 0.658 g /18 g/mol = 0.03656 moles
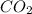 = 1.285 g
/44.01 g/mol = 0.029197 moles
= 1.285 g
/44.01 g/mol = 0.029197 moles


