Chemistry, 25.12.2019 01:31 zeesharpe05
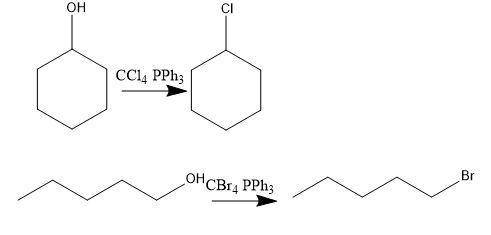
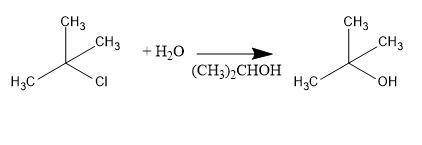
Consider the above reaction using 25.0 ml of tert-butyl alcohol (d = 0.786 g/ml) with 60.0 ml of concentrated hydrobromic acid (d = 1.49 g/ml, 47.0% hbr). on a separate sheet calculate the theoretical yield in grams and the percent yield for a reaction that produced 26.1 g of tert-butyl bromide. clearly show the set ups to determine the limiting reactant and other calculations using proper units and significant figures. consult your textbook for the following two synthesis. keep in mind that conc. hcl and conc. hbr will not give good yields of alkyl halides by the reaction with primary and secondary alcohols. give the balanced equation to prepare chlorocyclohexane in good yield from cyclohexanol. give the balanced equation for the preparation in good yield of 1-bromopentane from 1-pentanol. the reverse of the reaction you performed in the lab can also occur. under the proper conditions, tertiary alkyl halides may undergo a hydrolysis reaction to form an alcohol and a hydrogen halide. complete and balance the following equation. (remember the general ii experiment? )

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1


You know the right answer?
Consider the above reaction using 25.0 ml of tert-butyl alcohol (d = 0.786 g/ml) with 60.0 ml of con...
Questions


Arts, 14.12.2021 23:30

Mathematics, 14.12.2021 23:30

History, 14.12.2021 23:30

Social Studies, 14.12.2021 23:30

Mathematics, 14.12.2021 23:30


Chemistry, 14.12.2021 23:30

English, 14.12.2021 23:30




Mathematics, 14.12.2021 23:30

Mathematics, 14.12.2021 23:30

Mathematics, 14.12.2021 23:30

History, 14.12.2021 23:30

Social Studies, 14.12.2021 23:30

Mathematics, 14.12.2021 23:30


Chemistry, 14.12.2021 23:30