Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1


Chemistry, 22.06.2019 04:00
Which of the following ocean acidification? are the most likely side effects of a ph less than 7.0 in the ocean b. more metal salts altering the ocean chemistry c. dissolution of the shells of marine organisms d. both a & b e. all of the above.
Answers: 3

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1
You know the right answer?
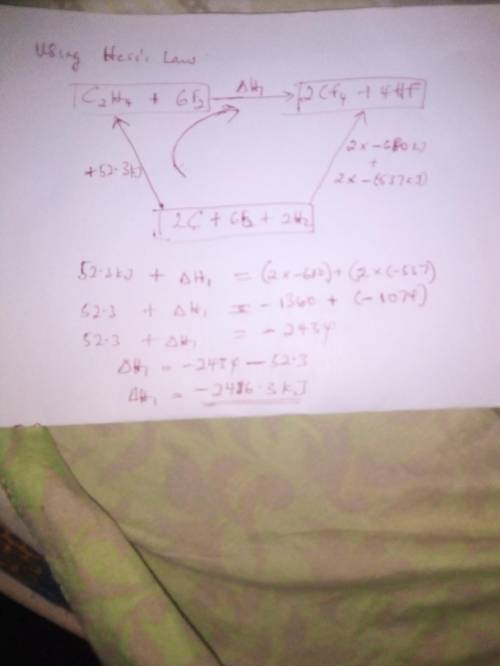
From the enthalpies of reactionh2 (g) + f2 (g) → 2 hf (g) δh = -537 kjc (s) + 2 f2 (g) → cf4 (g) δh...
Questions


History, 01.02.2021 22:20


Mathematics, 01.02.2021 22:20


Mathematics, 01.02.2021 22:20





Health, 01.02.2021 22:20


Mathematics, 01.02.2021 22:20


History, 01.02.2021 22:20



Mathematics, 01.02.2021 22:20

Mathematics, 01.02.2021 22:20

Mathematics, 01.02.2021 22:20