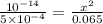Chemistry, 25.12.2019 06:31 rosehayden21
Calculate the ph at the equivalence point for the titration of 0.130 m methylamine (ch3nh2) with 0.130 m hcl. the k b kb of methylamine is 5.0 × 10 − 4 .

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1
You know the right answer?
Calculate the ph at the equivalence point for the titration of 0.130 m methylamine (ch3nh2) with 0.1...
Questions

SAT, 02.12.2020 19:00


Mathematics, 02.12.2020 19:00

English, 02.12.2020 19:00



Mathematics, 02.12.2020 19:00

Mathematics, 02.12.2020 19:00






History, 02.12.2020 19:00


French, 02.12.2020 19:00




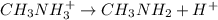
![K_a=\frac{K_w}{K_b} = \frac{[CH_3NH_2][H^+] }{CH_3NH_3^+}](/tpl/images/0432/6711/6bbed.png)