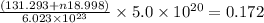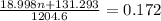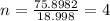Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3
You know the right answer?
Asample of a compound of xenon and fluorine contains molecules of a single type; xefn, where n is a...
Questions

History, 03.03.2021 01:10

Mathematics, 03.03.2021 01:10



Arts, 03.03.2021 01:10


English, 03.03.2021 01:10




Mathematics, 03.03.2021 01:10

Mathematics, 03.03.2021 01:10


Mathematics, 03.03.2021 01:10

Mathematics, 03.03.2021 01:10




Mathematics, 03.03.2021 01:10

 .
.
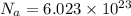
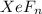 = 131.293+ n18.998 g
= 131.293+ n18.998 g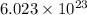 molecules have a mass of 131.293+ n18.998 g
molecules have a mass of 131.293+ n18.998 g molecules have a mass of
molecules have a mass of 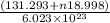 g
g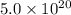 molecules have a mass of
molecules have a mass of 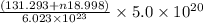 g
g