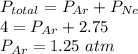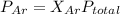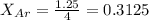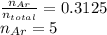Chemistry, 25.12.2019 20:31 michell200428
Agas mixture of ne and ar has a total pressure of 4.00 atm and contains 16.0 mol of gas. if the partial pressure of ne is 2.75 atm, how many moles of ar are in the mixture?
a) 11.0
b) 5.00
c) 6.75
d) 9.25
e) 12.0

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Agas mixture of ne and ar has a total pressure of 4.00 atm and contains 16.0 mol of gas. if the part...
Questions


English, 08.11.2020 03:10


Mathematics, 08.11.2020 03:10



Biology, 08.11.2020 03:10



Physics, 08.11.2020 03:10

Chemistry, 08.11.2020 03:10

Mathematics, 08.11.2020 03:10



Mathematics, 08.11.2020 03:10

Mathematics, 08.11.2020 03:10

English, 08.11.2020 03:10


Computers and Technology, 08.11.2020 03:10