
Chemistry, 25.12.2019 23:31 spacehunter22
An unknown weak acid, ha, it titrated with 1.2 m naoh. the ph at the halfway point of this titration was found to be 4.102. if the initial ph of the weak acid solution (before titration) has a ph of 2.308, what was the concentration of the weak acid solution?

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 00:00
What does an electron configuration for an atom relate to the atoms placement on the periodic table
Answers: 2

Chemistry, 23.06.2019 12:00
Jill is pushing a box across the floor. which represents the upward force perpendicular to the floor? a) fp b) ff c) fn d) fg
Answers: 1
You know the right answer?
An unknown weak acid, ha, it titrated with 1.2 m naoh. the ph at the halfway point of this titration...
Questions









Computers and Technology, 14.12.2019 04:31










Mathematics, 14.12.2019 04:31

Arts, 14.12.2019 04:31

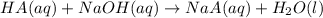
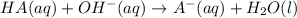
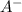 produced. That said, we have a weak acid and its conjugate base forming a buffer. We may apply the Henderson-Hasselbach equation for buffers here:
produced. That said, we have a weak acid and its conjugate base forming a buffer. We may apply the Henderson-Hasselbach equation for buffers here:![pH = pK_a + log(\frac{[A^-]}{[HA})](/tpl/images/0433/2346/a2dd4.png)
![[HA] = [A^-]](/tpl/images/0433/2346/96807.png) when half of equivalence volume of NaOH is added.
when half of equivalence volume of NaOH is added.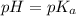 at midpoint. We may then find the acid ionization constant for this acid:
at midpoint. We may then find the acid ionization constant for this acid: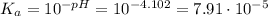
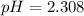
![pH = -log[H_3O^+]](/tpl/images/0433/2346/89cf7.png) , then
, then ![[H_3O^+] = 10^{-pH}](/tpl/images/0433/2346/341c1.png)
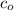 , we may write the equilibrium constant expression as:
, we may write the equilibrium constant expression as:![K_a = \frac{[H_3O^+]^2}{c_o - [H_3O^+]}](/tpl/images/0433/2346/86a10.png)
![c_o = \frac{[H_3O^+]^2}{K_a} + [H_3O^+] = \frac{10^{-2pH}}{K_a} + 10^{-pH} = \frac{10^{-2\cdot 2.308}}{7.91\cdot 10^{-5}} + 10^{-2.308} = 0.311 M](/tpl/images/0433/2346/5826b.png)


