
Chemistry, 26.12.2019 19:31 Tayannamorgan2373
What mass of this substance must evaporate to freeze 190 g of water initially at 17 ∘c? (the heat of fusion of water is 334 j/g; the specific heat of water is 4.18 j/(g⋅

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2


Chemistry, 23.06.2019 05:00
Match the term to its description match term definition chemical energy a) internal energy caused by vibrations of atoms and molecules electrical energy b) electromagnetic energy that travels in waves radiant energy c) the movement of an electrical charge thermal energy d) potential energy stored in the bonds between atoms
Answers: 1

Chemistry, 23.06.2019 09:00
A0.10 m aqueous solution of sodium sulfate is a better conductor of electricity than a 0.10 m aqueous solution of sodium chloride. which of the following best explains this observation? (a) sodium sulfate is more soluble in water than sodium chloride. (b) sodium sulfate has a higher molar mass than sodium chloride. (c) to prepare a given volume of 0.10 m solution, the mass of sodium sulfate needed is more than twice the mass of sodium chloride needed. (d) more moles of ions are present in a given volume of 0.10 m sodium sulfate than in the same volume of 0.10 m sodium chloride. (e) the degree of dissociation of sodium sulfate in solution is significantly greater than that of sodium chloride.
Answers: 2
You know the right answer?
What mass of this substance must evaporate to freeze 190 g of water initially at 17 ∘c? (the heat o...
Questions

Mathematics, 17.11.2020 20:30

Mathematics, 17.11.2020 20:30

History, 17.11.2020 20:30

Health, 17.11.2020 20:30



History, 17.11.2020 20:30

Mathematics, 17.11.2020 20:30


Geography, 17.11.2020 20:30





Chemistry, 17.11.2020 20:30

Mathematics, 17.11.2020 20:30

Mathematics, 17.11.2020 20:30

Computers and Technology, 17.11.2020 20:30


Mathematics, 17.11.2020 20:30

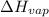 of
of 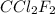 is 289 J/g. What mass of this substance must evaporate in order to freeze 190 g of water initially at 17 degrees C? (heat of fusion of water is 334 J/g; specific heat of water is 4.18 J/g.K).
is 289 J/g. What mass of this substance must evaporate in order to freeze 190 g of water initially at 17 degrees C? (heat of fusion of water is 334 J/g; specific heat of water is 4.18 J/g.K). ![\text{Total heat absorbed}=[m\times c_{p,l}\times (T_{final}-T_{initial})]+m\times \Delta H_{fusion}](/tpl/images/0433/9211/0a040.png)
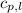 = specific heat of liquid water =
= specific heat of liquid water = 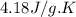
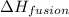 = enthalpy change for fusion of water =
= enthalpy change for fusion of water = 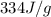
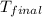 = final temperature =
= final temperature = 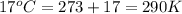
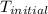 = initial temperature =
= initial temperature = 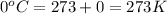
![\text{Total heat absorbed}=[190g\times 4.18J/g.K\times (290-273)K]+190g\times 334J/g](/tpl/images/0433/9211/78fd6.png)
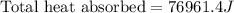
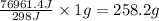 of
of 

