
Chemistry, 26.12.2019 20:31 portedon6626
Suppose a system returns to its original overall, internal energy ((∆u) after the following changes. in step 1, 25 j of work is done on the system as it releases 37 j of energy as heat. if, in step 2, the system performs 12 j of work, how much heat is transferred?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1


Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
Suppose a system returns to its original overall, internal energy ((∆u) after the following changes....
Questions

History, 23.07.2019 15:00




Mathematics, 23.07.2019 15:00


Mathematics, 23.07.2019 15:00

Chemistry, 23.07.2019 15:00

Mathematics, 23.07.2019 15:00

Mathematics, 23.07.2019 15:00


Mathematics, 23.07.2019 15:00

Chemistry, 23.07.2019 15:00


Mathematics, 23.07.2019 15:00

Mathematics, 23.07.2019 15:00

Biology, 23.07.2019 15:00

Mathematics, 23.07.2019 15:00

Physics, 23.07.2019 15:00

History, 23.07.2019 15:00

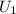 = -37 + 1.25 = -35.75 J
= -37 + 1.25 = -35.75 J

