
Chemistry, 26.12.2019 21:31 brandonthomas11
A2.5 l flask is filled with 0.25 atm so3, 0.20 atm so2, and 0.40 atm o2, and allowed to reach equilibrium. assume at the temperature of the mixture is chosen so that kp = 0.12. predict the effect on the partial pressure of so3 as equilibrium is achieved by using q, the reaction quotient.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

Chemistry, 23.06.2019 10:30
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 3.75 mol fe and 8.70 mol nio(oh) react?
Answers: 1
You know the right answer?
A2.5 l flask is filled with 0.25 atm so3, 0.20 atm so2, and 0.40 atm o2, and allowed to reach equili...
Questions






English, 19.03.2020 06:20

Health, 19.03.2020 06:20



History, 19.03.2020 06:20


Mathematics, 19.03.2020 06:20






Social Studies, 19.03.2020 06:21


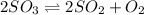
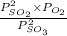
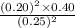
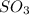 as equilibrium is achieved by using Q, is as follows.
as equilibrium is achieved by using Q, is as follows.

