Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1
You know the right answer?
Consider the following reaction: a2 + b2 → 2ab δh = –321 kj bond energy (a2) = 1/2ab bond energy (b...
Questions



History, 08.10.2019 06:30

Mathematics, 08.10.2019 06:30


English, 08.10.2019 06:30

Chemistry, 08.10.2019 06:30

English, 08.10.2019 06:30

Mathematics, 08.10.2019 06:30


Mathematics, 08.10.2019 06:30

Health, 08.10.2019 06:30


English, 08.10.2019 06:30

Mathematics, 08.10.2019 06:30





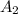 is -238 kJ/mol
is -238 kJ/mol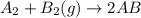

![\Delta H=\sum [n\times B.E(reactant)]-\sum [n\times B.E(product)]](/tpl/images/0434/0215/42942.png)
![\Delta H=[(n_{A_2}\times B.E_{A_2})+(n_{B_2}\times B.E_{B_2}) ]-[(n_{AB}\times B.E_{AB})]](/tpl/images/0434/0215/c021c.png)
![\Delta H=[(1\times x)+(1\times B.E_{B_2}) ]-[(2\times 2x)]](/tpl/images/0434/0215/7aa1f.png)
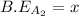
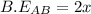
![-321=[(1\times x)+(1\times 393)]-[(2\times 2x)]](/tpl/images/0434/0215/06819.png)