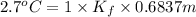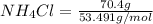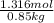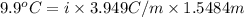When of benzamide are dissolved in of a certain mystery liquid , the freezing point of the solution is less than the freezing point of pure . calculate the mass of ammonium chloride that must be dissolved in the same mass of to produce the same depression in freezing point. the van't hoff factor for ammonium chloride in .

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:00
How many moles of magnesium is 3.01 x10^22 atoms of magnesium?
Answers: 1


Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
When of benzamide are dissolved in of a certain mystery liquid , the freezing point of the solution...
Questions

Mathematics, 22.07.2019 05:30



History, 22.07.2019 05:30

Spanish, 22.07.2019 05:30





History, 22.07.2019 05:30



Mathematics, 22.07.2019 05:30

English, 22.07.2019 05:30

Mathematics, 22.07.2019 05:30




Biology, 22.07.2019 05:30

Mathematics, 22.07.2019 05:30

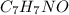 ) are dissolved in 850 g of a certain mystery liquid X, the freezing point of the solution is
) are dissolved in 850 g of a certain mystery liquid X, the freezing point of the solution is 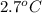 lower than the freezing point of pure X. On the other hand, when 70.4 g of ammonium chloride (
lower than the freezing point of pure X. On the other hand, when 70.4 g of ammonium chloride (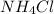 ) are dissolved in the same mass of X, the freezing point of the solution is
) are dissolved in the same mass of X, the freezing point of the solution is 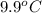 lower than the freezing point of pure X.
lower than the freezing point of pure X.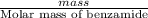
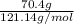
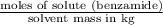
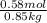
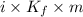 ,
, 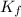 = freezing point constant of solvent
= freezing point constant of solvent