
Chemistry, 26.12.2019 22:31 DavidsonSaid
The acid dissociation constant ka of boric acid (h3bo3) is 5.8 times 10^-10. calculate the ph of a 4.4 m solution of boric acid. round your answer to 1 decimal place.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 14:00
What term describes technology that operates on an atomic level
Answers: 2

Chemistry, 23.06.2019 10:30
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh.the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3
You know the right answer?
The acid dissociation constant ka of boric acid (h3bo3) is 5.8 times 10^-10. calculate the ph of a 4...
Questions

Biology, 26.06.2021 21:40


Arts, 26.06.2021 21:40

Mathematics, 26.06.2021 21:40



Mathematics, 26.06.2021 21:40




English, 26.06.2021 21:50

Mathematics, 26.06.2021 21:50

French, 26.06.2021 21:50




Mathematics, 26.06.2021 21:50


English, 26.06.2021 21:50

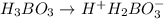
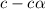
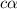
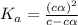
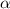 = ?
= ?
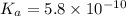
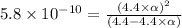
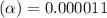
![[H^+]=c\times \alpha](/tpl/images/0434/0871/4fc41.png)
![[H^+]=4.4\times 0.000011=4.8\times 10^{-5}M](/tpl/images/0434/0871/865ab.png)
![pH=-log[H^+]](/tpl/images/0434/0871/15713.png)
![pH=-log[4.8\times 10^{-5}]=4.3](/tpl/images/0434/0871/ee066.png)
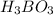 solution is 4.3
solution is 4.3


