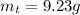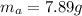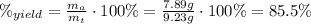Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:40
Achange in the number of neutrons in an atom will change an blank . when the number of protons changes in an atom, a new element will form.
Answers: 2


Chemistry, 22.06.2019 14:20
You have a liquid that exhibits diltancy. you want to pour it from a bottle. what should you do to the bottle before pouring
Answers: 1

Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Areaction with a theoretical yield of 9.23 g produced 7.89 g of product. what is the percent yield f...
Questions

History, 04.02.2020 11:50

Mathematics, 04.02.2020 11:50

Mathematics, 04.02.2020 11:50


Spanish, 04.02.2020 11:50

History, 04.02.2020 11:50




Arts, 04.02.2020 11:50


Mathematics, 04.02.2020 11:50

History, 04.02.2020 11:50

Physics, 04.02.2020 11:50


Chemistry, 04.02.2020 11:50




Physics, 04.02.2020 11:50