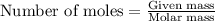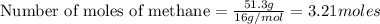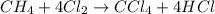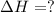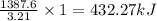Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

You know the right answer?
Methane (ch4) reacts with cl2 to yield ccl4 and hcl by the following reaction equation: ch4 + 4 cl2...
Questions


Biology, 02.10.2019 03:30


Mathematics, 02.10.2019 03:30


Social Studies, 02.10.2019 03:30







Geography, 02.10.2019 03:30


English, 02.10.2019 03:30




Physics, 02.10.2019 03:30

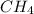 reacts with excess
reacts with excess 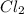 to yield 1387.6 kJ is 432.27kJ
to yield 1387.6 kJ is 432.27kJ