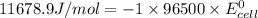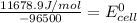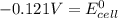Chemistry, 26.12.2019 23:31 BurwinkelElla19
Calculate the standard cell potential (e∘) for the reaction x(s)+y+(aq)→x+(aq)+y(s) if k = 8.97×10−3. express your answer to three significant figures and include the appropriate units. view available hint(s)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What mass of carbon dioxide is produced from the complete combustion of 4.50×10−3 g of methane?
Answers: 2

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1
You know the right answer?
Calculate the standard cell potential (e∘) for the reaction x(s)+y+(aq)→x+(aq)+y(s) if k = 8.97×10−3...
Questions


Mathematics, 12.03.2020 16:12




Mathematics, 12.03.2020 16:12


Social Studies, 12.03.2020 16:12



English, 12.03.2020 16:13

Mathematics, 12.03.2020 16:13

Mathematics, 12.03.2020 16:13

Mathematics, 12.03.2020 16:13


Mathematics, 12.03.2020 16:13

Mathematics, 12.03.2020 16:13

Mathematics, 12.03.2020 16:13

English, 12.03.2020 16:13

Mathematics, 12.03.2020 16:13

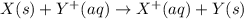 is -0.121 V
is -0.121 V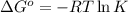
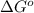 = Standard Gibbs free energy = ?
= Standard Gibbs free energy = ?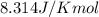
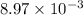
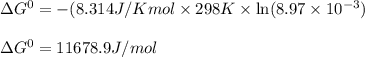
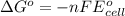
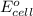 = standard cell potential = ?
= standard cell potential = ?