Chemistry, 27.12.2019 00:31 alexisthegirl
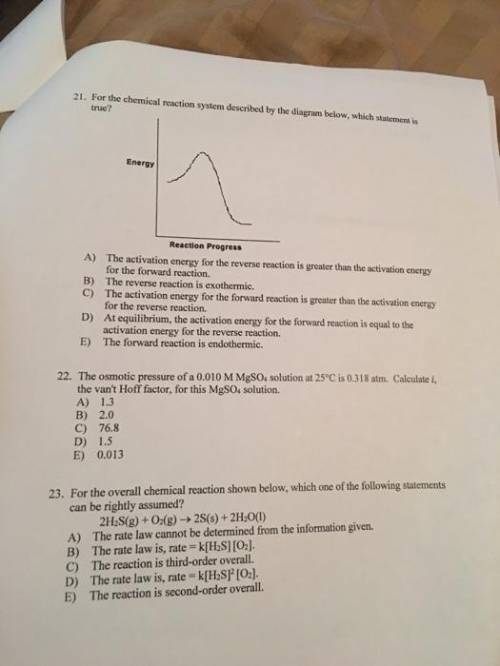
For the chemical reaction system described by the diagram below, which statement is true? picture the forward reaction is endothermic. the activation energy for the forward reaction is greater than the activation energy for the reverse reaction. at equilibrium, the activation energy for the forward reaction is equal to the activation energy for the reverse reaction. the activation energy for the reverse reaction is greater than the activation energy for the forward reaction. the reverse reaction is exothermic.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3

You know the right answer?
For the chemical reaction system described by the diagram below, which statement is true? picture t...
Questions

Mathematics, 31.01.2020 09:46




History, 31.01.2020 09:46

Mathematics, 31.01.2020 09:46

Biology, 31.01.2020 09:46


Mathematics, 31.01.2020 09:46

History, 31.01.2020 09:46


Biology, 31.01.2020 09:46





History, 31.01.2020 09:46

Biology, 31.01.2020 09:46

Mathematics, 31.01.2020 09:46

Mathematics, 31.01.2020 09:46