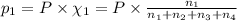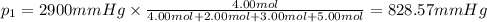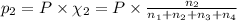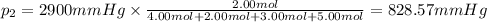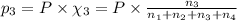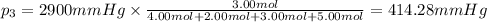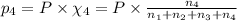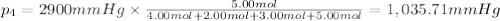Chemistry, 27.12.2019 02:31 ahmedeldyame
Amixture of gases consists of 4.00 moles of he, 2.00 moles of h2, 3.00 moles of co2 and 5.00 moles of ar. the total pressure of the mixture is 2900 mm. determine the mole fraction of each gas in the mixture. determine the mole percent of each gas in the mixture. determine the partial pressure of each gas in the mixture.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

You know the right answer?
Amixture of gases consists of 4.00 moles of he, 2.00 moles of h2, 3.00 moles of co2 and 5.00 moles o...
Questions


Computers and Technology, 01.02.2021 17:00




Mathematics, 01.02.2021 17:00















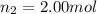
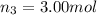
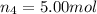

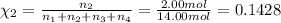
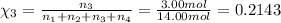
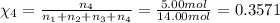
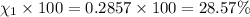
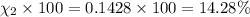
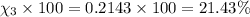
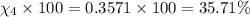
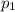
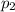

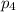
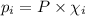
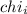 = Mole fraction of ith component
= Mole fraction of ith component