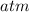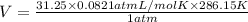Chemistry, 27.12.2019 02:31 salutemeimchloe
Combustion of hydrocarbons such as methane (ch) produces carbon dioxide, a "greenhouse gas." greenhouse gases in the earths atmosphere can trap the sun's heat, raising the average temperature of the earth. for this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. write a balanced chemical equation, including physical state symbols, for the iston of ous methane ito gaseous carbon donde and gaseous water 2. suppose 0.500 kg of methane are burned in air at a pressure of exactly 1 atm and temperatue of 13.0 °c. caoate the volume of carbon dode gas that is produced. be sure your answer has the correct number of significant digits

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
Combustion of hydrocarbons such as methane (ch) produces carbon dioxide, a "greenhouse gas." greenho...
Questions

Biology, 15.11.2019 18:31

Biology, 15.11.2019 18:31


Chemistry, 15.11.2019 18:31

Mathematics, 15.11.2019 18:31

Mathematics, 15.11.2019 18:31

Mathematics, 15.11.2019 18:31


English, 15.11.2019 18:31


Mathematics, 15.11.2019 18:31










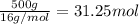
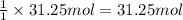 of carbon dioxide
of carbon dioxide