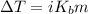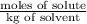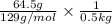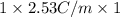Chemistry, 27.12.2019 03:31 douglasally
Assuming ideal behavior, calculate the boiling point in c of solution that contains 64.5g or the non-volatile non-electrolyte quinoline( molar mass= 129g/mole) in 500 grams of benzene. for benzene the normal boling point is 80.10 c and kb = 2.53c/m

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2

You know the right answer?
Assuming ideal behavior, calculate the boiling point in c of solution that contains 64.5g or the non...
Questions





Mathematics, 19.06.2021 14:00

Mathematics, 19.06.2021 14:00

History, 19.06.2021 14:00

Mathematics, 19.06.2021 14:00

Mathematics, 19.06.2021 14:00


Chemistry, 19.06.2021 14:00

Mathematics, 19.06.2021 14:00

Mathematics, 19.06.2021 14:00


English, 19.06.2021 14:00

English, 19.06.2021 14:00



English, 19.06.2021 14:00

Mathematics, 19.06.2021 14:00

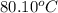 ,
, 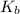 = 2.53 C/m
= 2.53 C/m