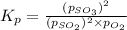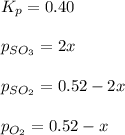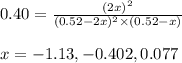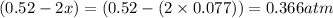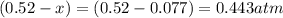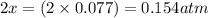Chemistry, 27.12.2019 03:31 IIHarmonyII
At 920 k, kp = 0.40 for the following reaction. 2 so2(g) + o2(g) equilibrium reaction arrow 2 so3(g) calculate the equilibrium partial pressures of so2, o2, and so3 produced from an initial mixture in which the partial pressures of so2 and o2 = 0.52 atm and the partial pressure of so3 = 0 (exactly).

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Select each correct answer. more than one answer may be correct. which of the following is a characteristic of unicellular organisms? they can possess tissues and organs. all of their functions are performed by a single cell. they are usually microscopic. each of their cells is specialized to perform a specific function.
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1
You know the right answer?
At 920 k, kp = 0.40 for the following reaction. 2 so2(g) + o2(g) equilibrium reaction arrow 2 so3(g)...
Questions



Social Studies, 26.10.2020 22:30

History, 26.10.2020 22:30


English, 26.10.2020 22:30




Mathematics, 26.10.2020 22:30

Health, 26.10.2020 22:30



History, 26.10.2020 22:30



Mathematics, 26.10.2020 22:30

Biology, 26.10.2020 22:30

Mathematics, 26.10.2020 22:30

Health, 26.10.2020 22:30

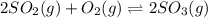
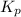 for above equation follows:
for above equation follows: